Synthesis process
Pharmaceutical industry
PROCESS
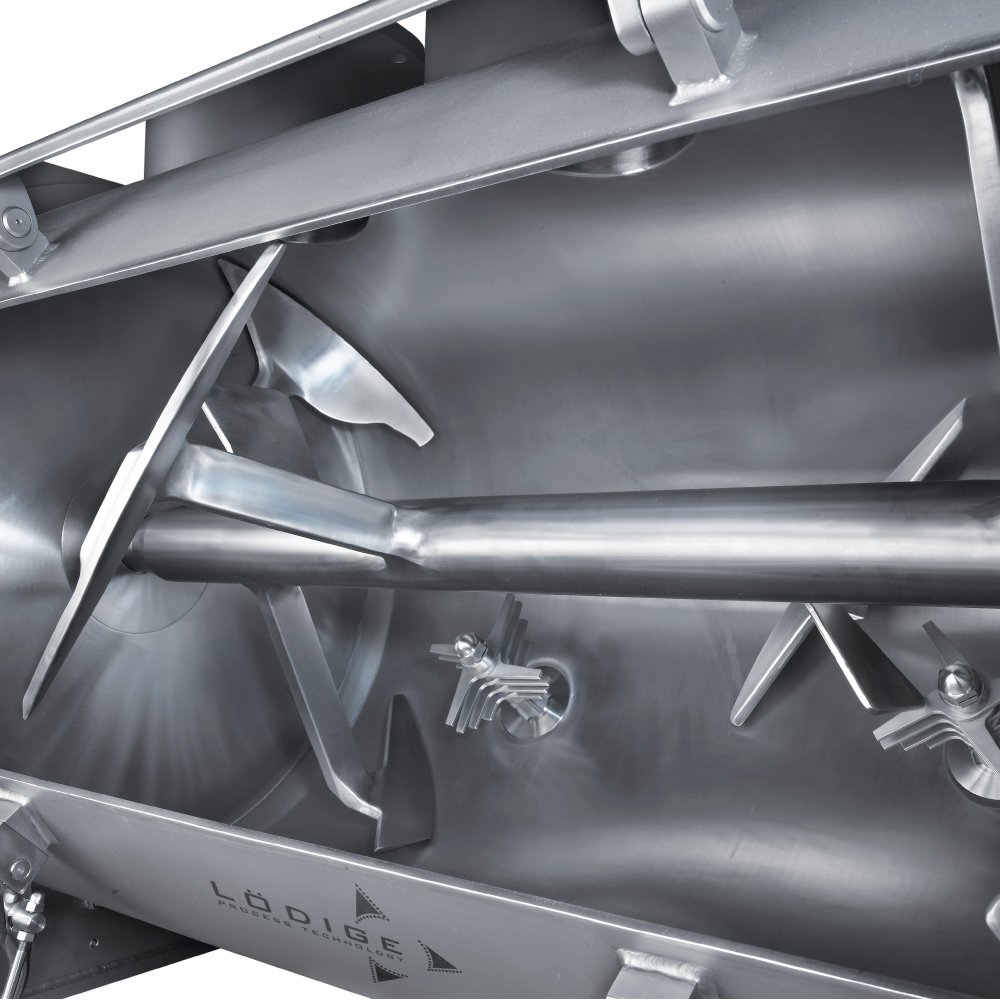
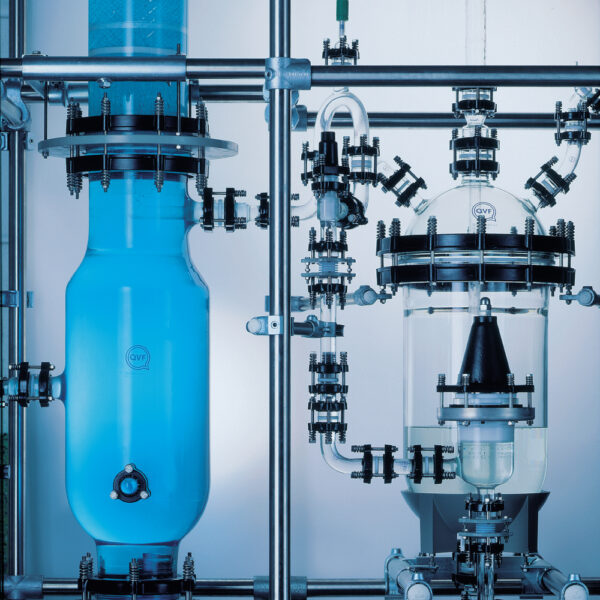
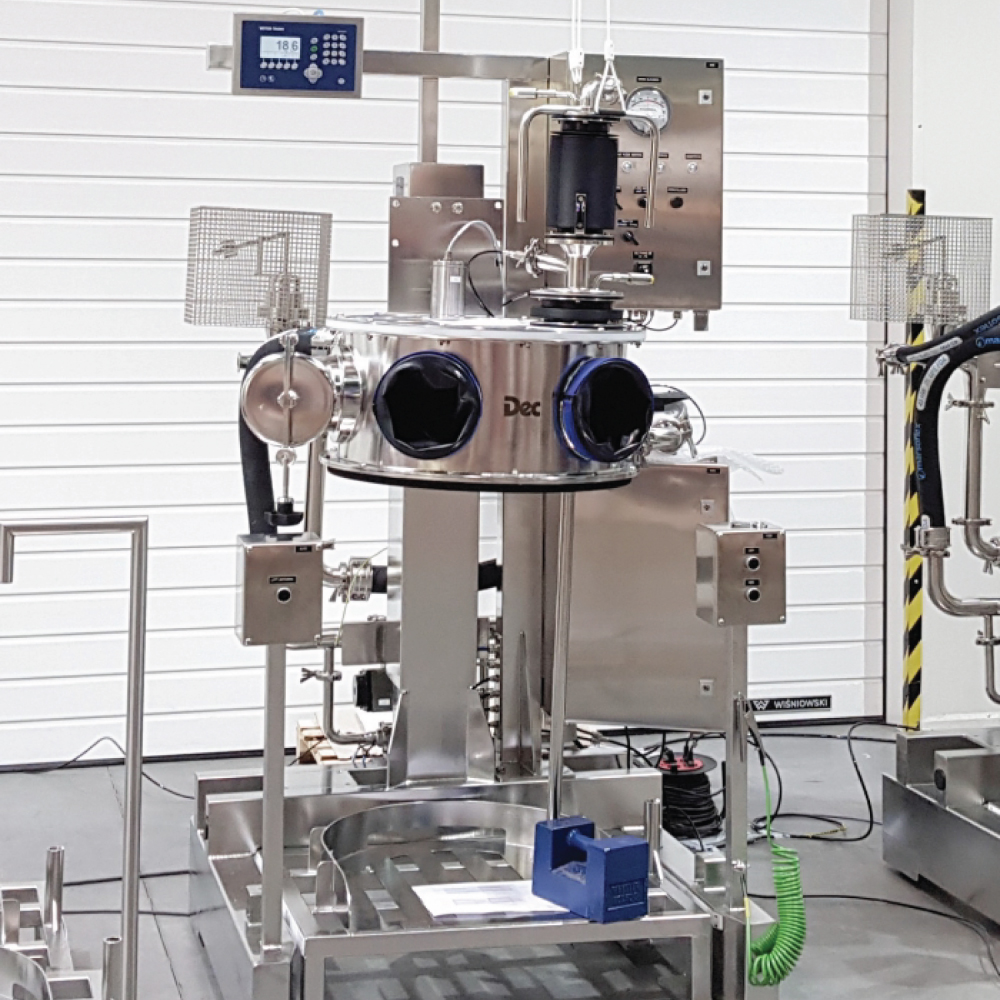
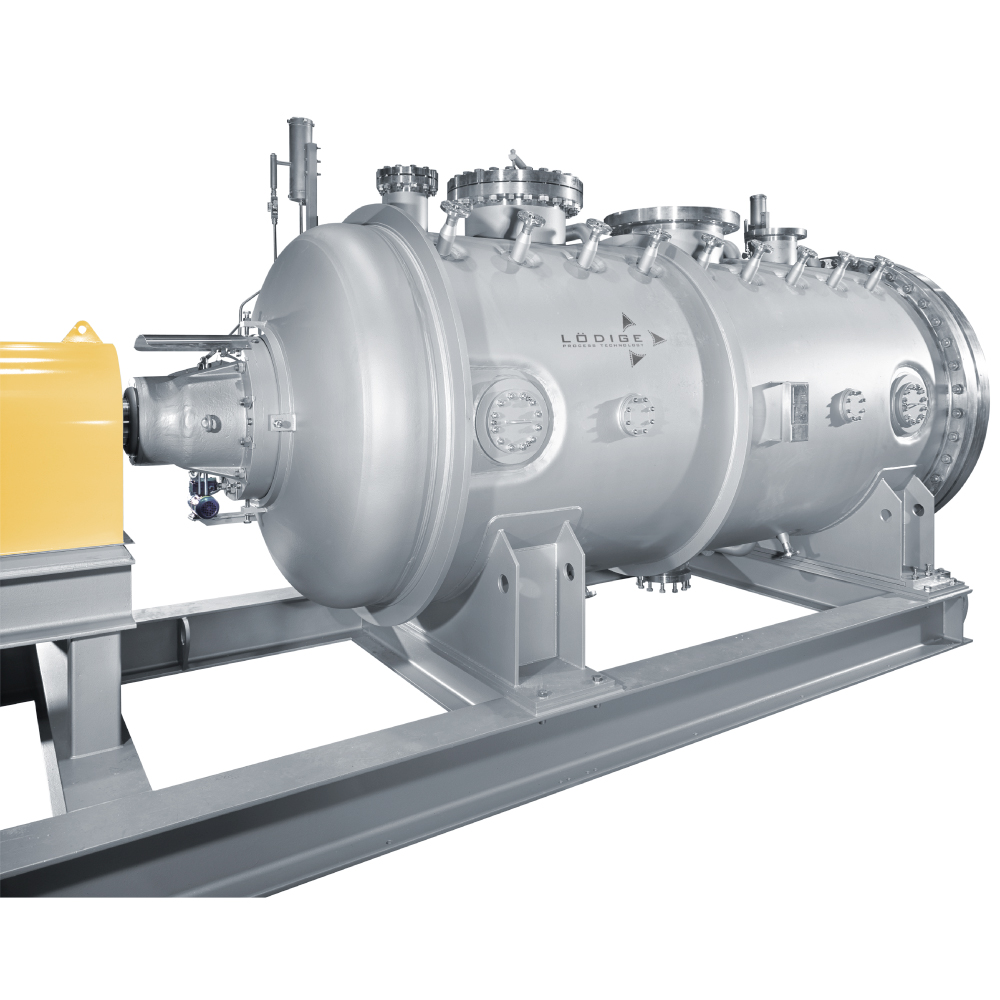
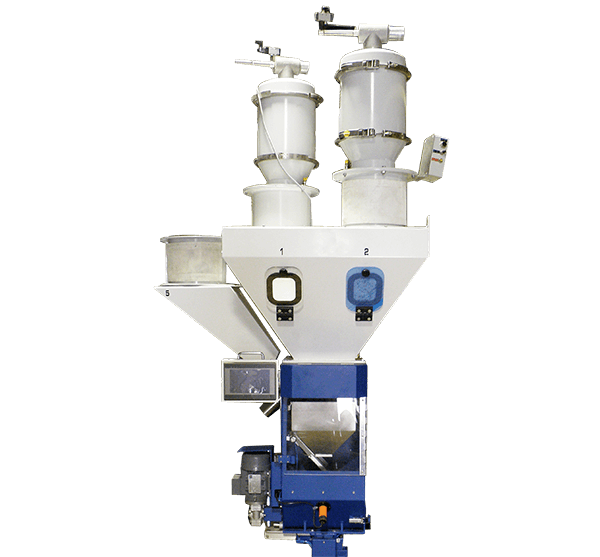
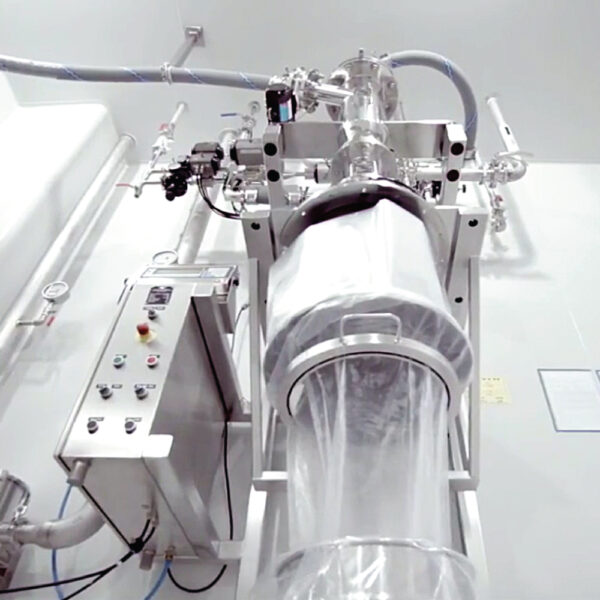
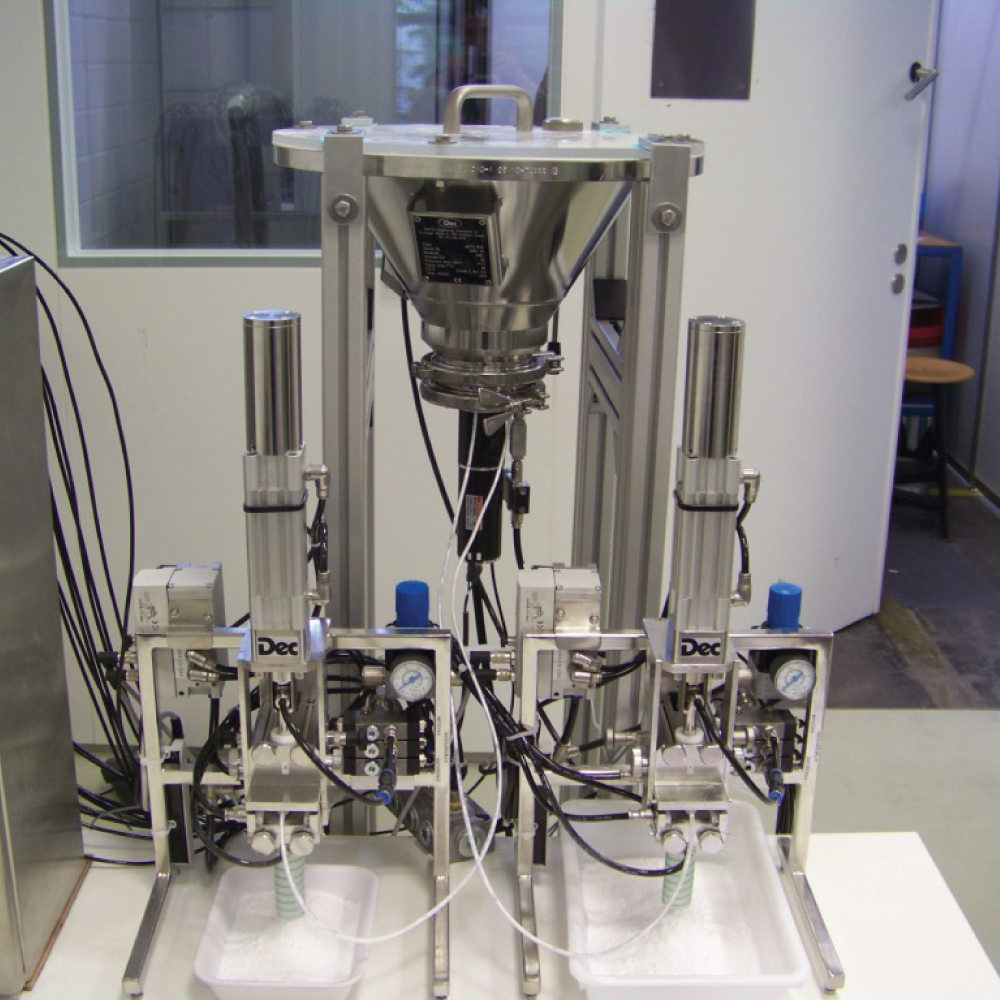
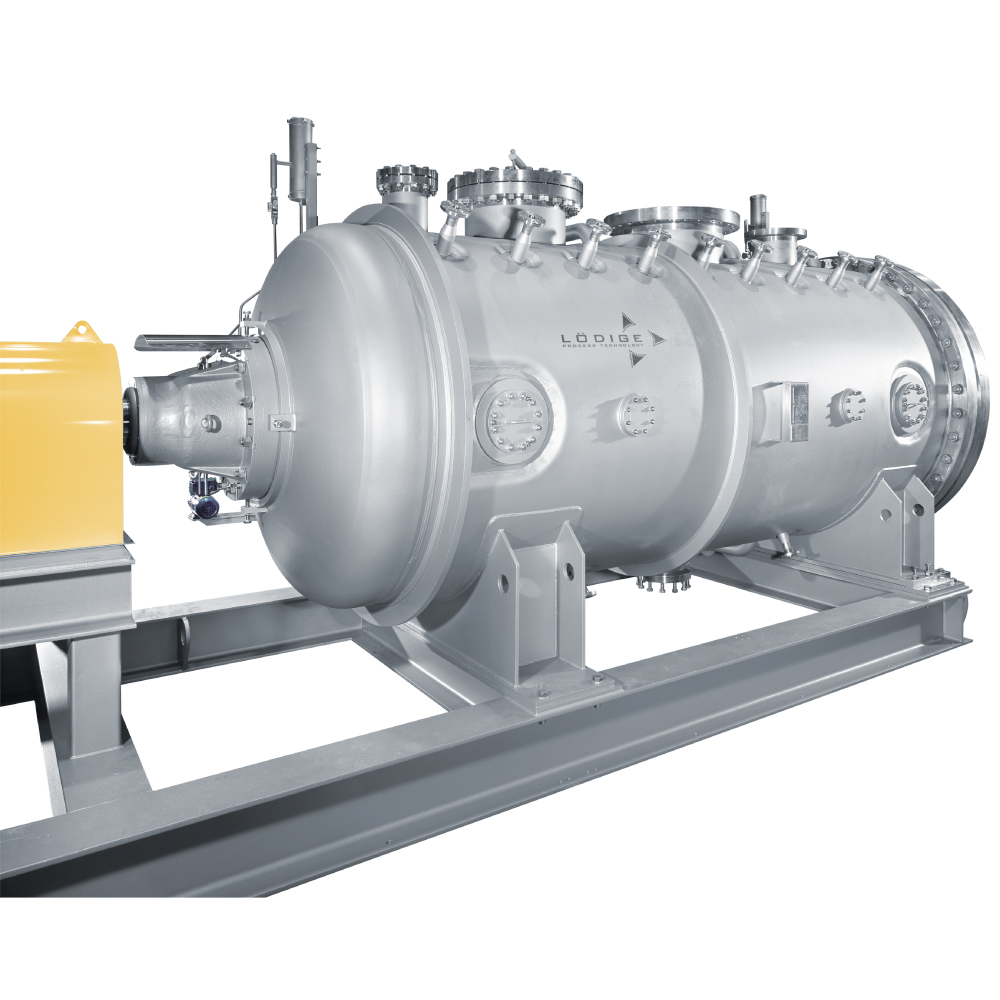
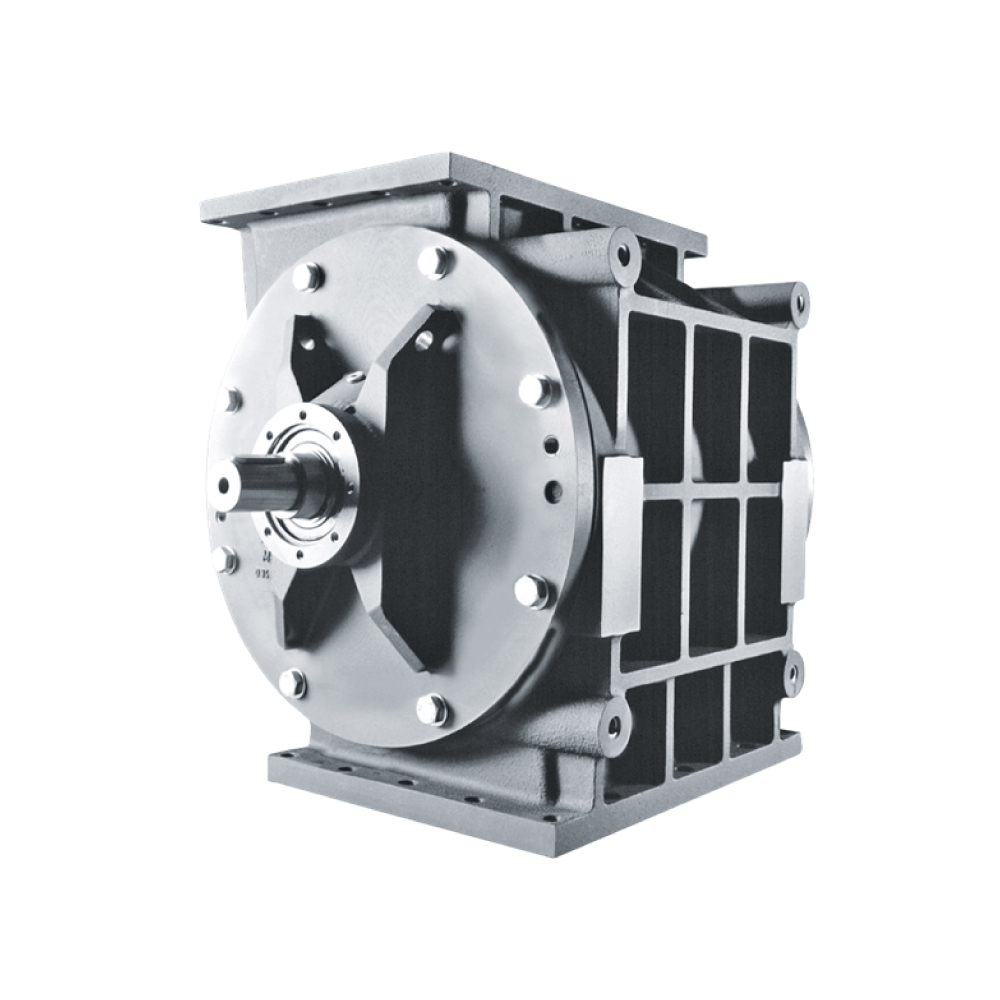
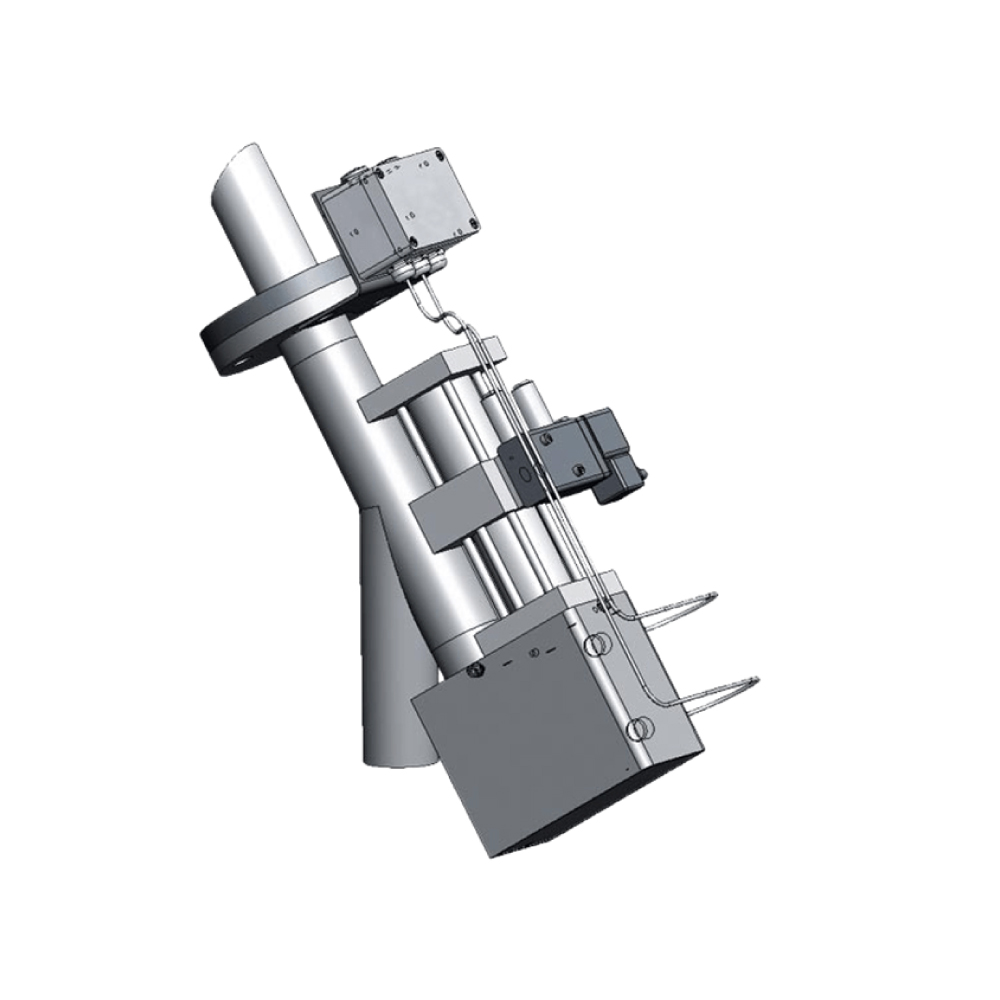
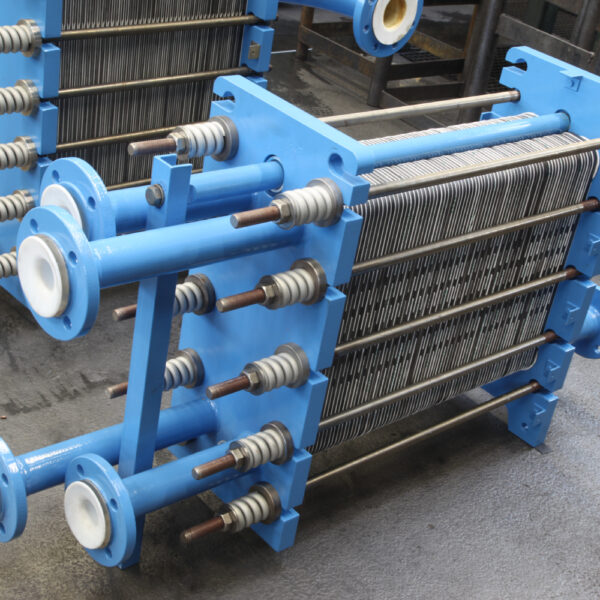
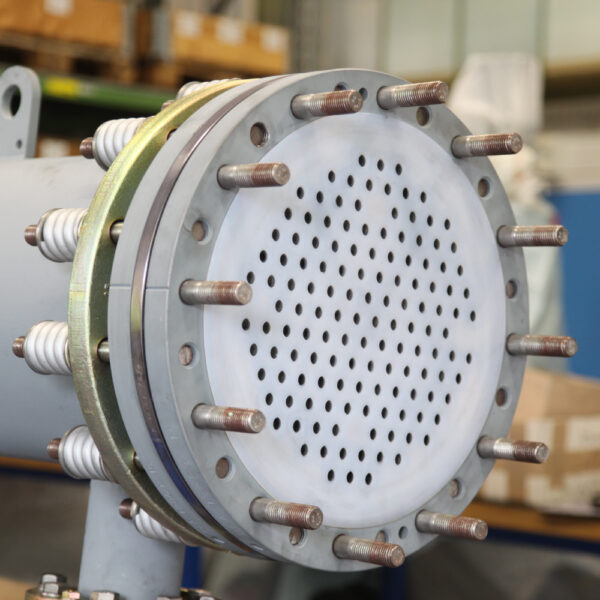
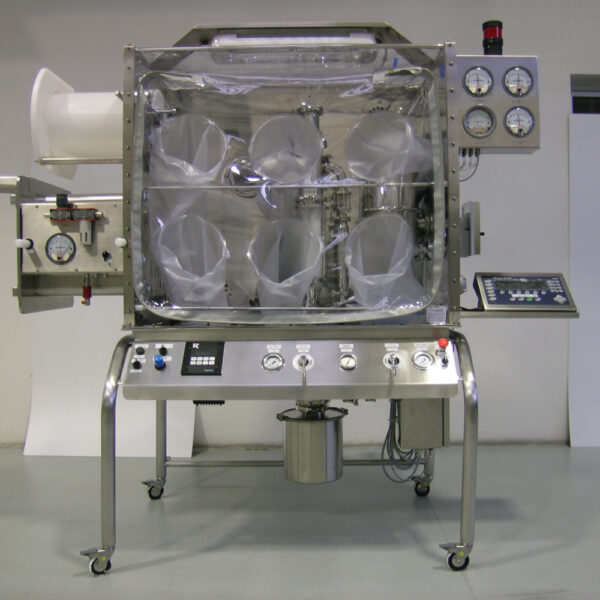
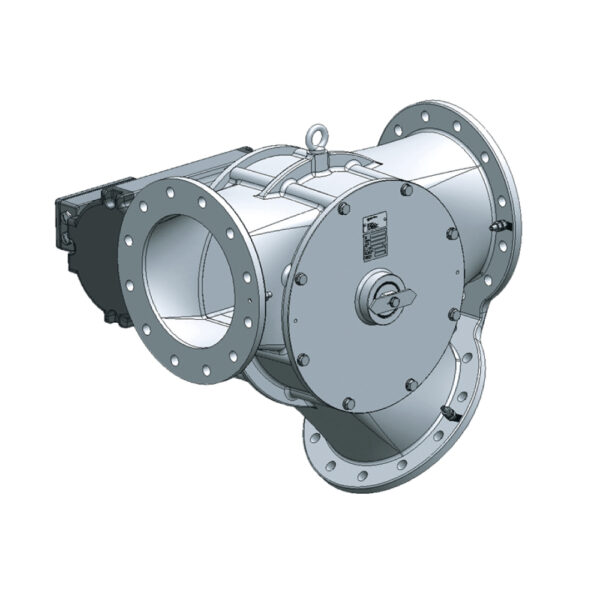
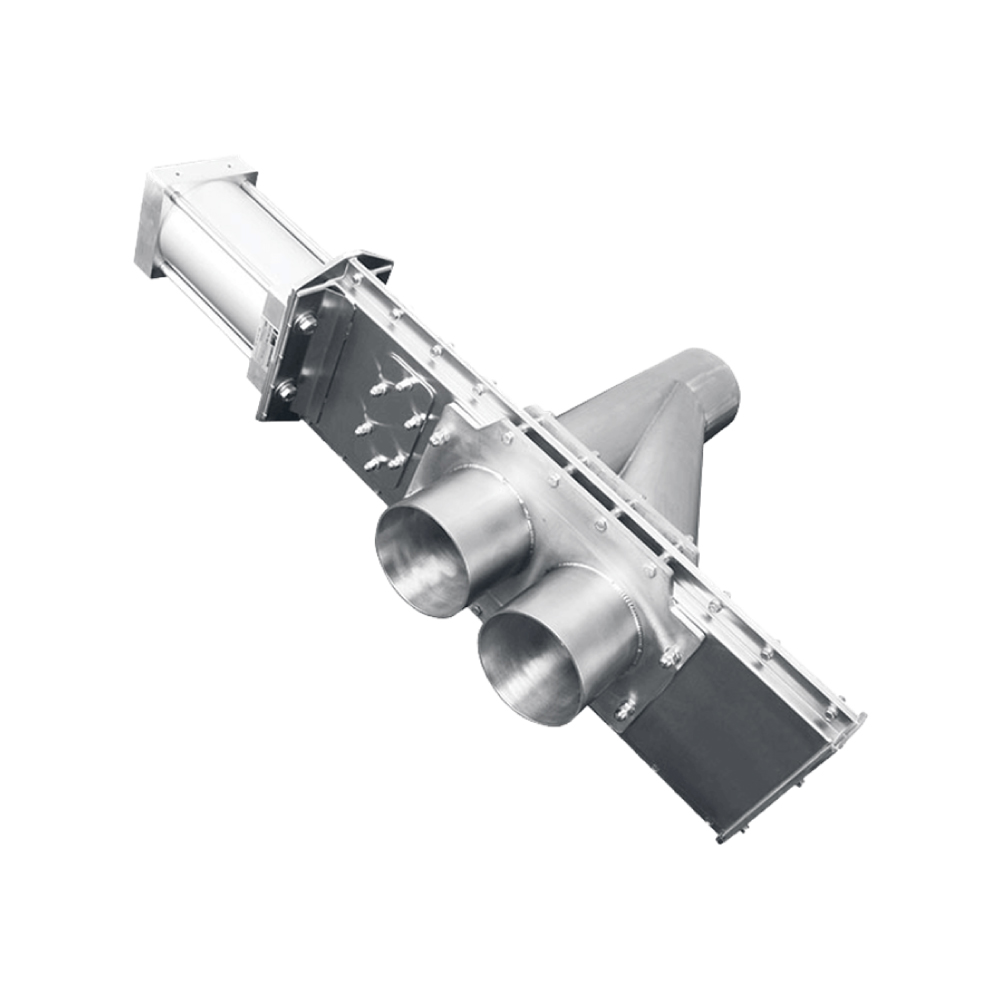
API (active pharmaceutical ingredients) production typically uses a synthesis process, encompassing raw material storage, dispensing, and charging into reactors, followed by dryings steps using filter dryers, centrifuges, or vacuum dryers. The product is then conveyed, micronized, dispensed, and packed off. These steps normally require secure containment, depending on the nature of the API, using isolators, powder transport systems, and adapted valves.
CHALLENGES
Healthcare reforms, patent expiries and increased service requirements are forcing pharmaceutical companies to adapt their business models. All pharma companies are facing a new landscape that includes challenges such as meeting complex regulatory legal frameworks, increased market competitiveness, higher containment requirements for more potent APIs, as well as demands for multi-purpose processes, lean production methods, and decreased maintenance costs.
EQUIPMENT
Together with well-established partners, Thurne can supply the right high-quality process equipment for material handling solutions, equipment for dispensing, and complete process lines. This includes contained discharge, filling, conveying, dosing, mixing, dispensing, sampling, micronizing, milling, sieving, granulation, and pack off equipment. All equipment is designed for high containment applications and follows the cGMP standards.
Products
Product ARTICLES
Download PHARMACEUTICAL PDF
Honesty, openness and respect earns trust. Trust equals reputation.
Acting with these five core values in mind we aim to bring an all-important “human touch” to the technological world we work in.
Product SUPPLIERS
Our CONTACTS
If you have any questions please don`t hesitate to contact Thurne specialist directly:
Thurne Baltic

Phone number
Direct phone: +371 6616 3763 Mobile phone: +371 2689 6799Thurne Denmark


Phone number
Direct phone: +45 31 14 88 62
Phone number
Direct phone: +45 89 88 35 00 Mobile phone: +45 31 61 81 88Thurne Finland


Phone number
Mobile phone: +358 40 869 7736
Phone number
Mobile phone: +358 40 730 8464
Phone number
Mobile phone: +358 45 110 2337
Phone number
Mobile phone: +358 40 183 5870Thurne Sweden, Norway

Phone number
Direct phone: +46 8 55 76 93 48 Mobile phone: +46 76 516 72 37
Phone number
Direct phone: +46 765 172 007 Mobile phone: +46 8 5576 9347
Phone number
Direct phone: +46 8 557 693 46 Mobile phone: +46 76 517 23 83
Phone number
Direct phone: +46 (0) 735985448
Phone number
Direct phone: +46 (0)8 - 55 76 93 37 Mobile phone: +46 70 781 37 80
Phone number
Direct phone: +46 8 5576 9333 Mobile phone: +46 709 326 731
Phone number
Mobile phone: +358 40 183 5870
Phone number
Direct phone: +46 8 5576 9332 Mobile phone: +46 709 535 018Thurne Poland